BACKGROUND:
Acute Lymphoblastic leukemia (ALL) has good prognosis when treated with multiagent chemotherapy in pediatrics and young adolescents. Treatment of relapsed/refractory (R/R) ALL remains a challenge. Even after stem cell transplantation (SCT), the prognosis of R/R ALL is still grave. Chimeric antigen receptor- T cell (CAR-T) therapy, uses T cells engineered for cancer therapy. CD-19 specific Car-T cell is a recent advancement, FDA approved use of tisagenlecleucel in 2017 for R/R- B cell ALL in patients under 25years of age. In this systematic review we will discuss efficacy and safety of CD-19 specific CAR-T cell therapy in R/R B-ALL in pediatrics and young adults. There are still 30 clinical trials that are going on the CD-19 CAR-T cell therapy in R/R ALL in pediatrics and adults
MATERIALS and METHODS:
We searched PubMed, Embase, Clinical Trials, Web of Science and Cochrane. We searched without any filters and used Mesh Terms for "ALL" and "Chimeric antigen receptor". After screening of 2381 articles, we included 12 clinical trial and 3 prospective studies evaluating the role of CD-19 specific CAR-T cell in Relapsed/ Refractory ALL in pediatrics and young adults under 30years only. We followed PRISMA guidelines in literature search and selection of studies. We used "R" for meta-analysis.
RESULTS:
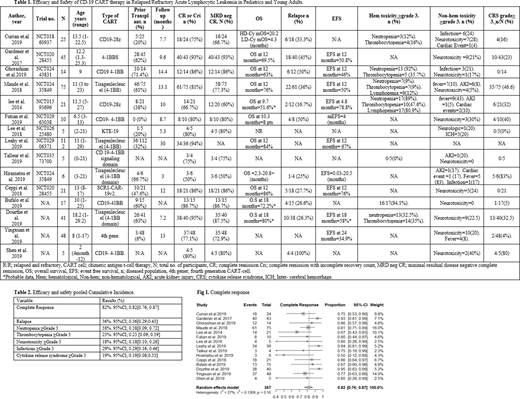
A total of 448 patients received a CD-19 specific CAR-T cell product and 446 patients were evaluable. The age range was 0-30 years. The female population in reported studies was 42.8% (n=111/259). Fludarabine and cyclophosphamide lymphodepleting therapy was used as a conditioning regimen followed by a single infusion of CAR-T cell product. Second generation CAR-T cell with a 4-1BB signaling domain was used in 66.7% of studies (n=10/15). High Risk cytogenetics was seen range from 4%-32% (n=53/220) and CNS disease in 66.9% (n=73/109) of the population. Median number of prior therapies ranges from 1 to 8 and 43.5%(n=186/247) had previous allo-HSCT. The median follow-up ranges from 3 to 14.4months. [Table 1]
Complete remission (CR) and complete remission with incomplete count recovery (CRi) range from 50%-95% of the total participants. CR with minimal residual disease (MRD) negative status was reported in 50% to 86% of total participants. The Relapse rate range from 26%-100% of the total participants. Of 82 cases of relapse, 27 had CD19 positive disease, 42 had CD19 negative, 10 had unknown status. There were 3 AML transformations. Median overall survival at 12months ranges from 63%-84%. Median event free survival ranges from 46%-76%. [Table 1]
The cumulative incidence of complete remission is 82% (heterogeneity,I2=27%) (95%CI; 0.82[0.76; 0.87]). Cumulative incidence of relapse after CD19 CAR-T cell therapy is 36% (heterogeneity,I2=10%), (95%CI; 0.36[0.29;0.43]). Similarly pooled cumulative incidence of ≥Grade 3 adverse events of neutropenia, thrombocytopenia, neurotoxicity, infections and cytokine release syndrome was 38%(95%CI; 0.38[0.09; 0.72]), 23%(95%CI; 0.23 [0.09; 0.39]), 18%(95%CI; 0.18[0.10; 0.26]) , 29%(95%CI; 0.29[0.16; 0.46]),19%(95%CI; 0.19[0.08;0.33]) respectively. [Table 2, Fig 1]
CONCLUSION:
CAR-T cell therapy against R/R B-ALL can achieve CR in significant pediatric patient population. The relapse rate is also high, about 36% pooled cumulative incidence. Being a bridging therapy, there is a need for additional therapy such as HSCT or maintenance targeted chemotherapy after CAR-T cell therapy while the patient is in remission. While most studies are phase-1 and there are still 30 ongoing clinical trials, we will be in a better position in near future to evaluate the effect of CAR-T cell therapy on overall survival and relapse rate after CAR-T cell therapy.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal